Discovering versatile biocatalysis that can be used to accelerate the synthesis of drugs and agrochemicals.
Based in the Frick Chemistry Laboratory at Princeton University, the Hyster lab integrates the fields of organic synthesis, organometallic chemistry, chemical biology, and protein engineering to develop new biocatalytic reactions that solve longstanding selectivity challenges and expand how enzymes can be used to achieve sustainable chemical synthesis.
Recent publications.
-

From Ground-State to Excited-State Activation Modes: Flavin-Dependent “Ene”-Reductases Catalyzed Non-natural Radical Reactions
Acc. Chem. Res. 2024, Article ASAP.
Publication Abstract
Enzymes are desired catalysts for chemical synthesis, because they can be engineered to provide unparalleled levels of efficiency and selectivity. Yet, despite the astonishing array of reactions catalyzed by natural enzymes, many reactivity patterns found in small molecule catalysts have no counterpart in the living world. With a detailed understanding of the mechanisms utilized by small molecule catalysts, we can identify existing enzymes with the potential to catalyze reactions that are currently unknown in nature. Over the past eight years, our group has demonstrated that flavin-dependent “ene”-reductases (EREDs) can catalyze various radical-mediated reactions with unparalleled levels of selectivity, solving long-standing challenges in asymmetric synthesis.
This Account presents our development of EREDs as general catalysts for asymmetric radical reactions. While we have developed multiple mechanisms for generating radicals within protein active sites, this account will focus on examples where flavin mononucleotide hydroquinone (FMNhq) serves as an electron transfer radical initiator. While our initial mechanistic hypotheses were rooted in electron-transfer-based radical initiation mechanisms commonly used by synthetic organic chemists, we ultimately uncovered emergent mechanisms of radical initiation that are unique to the protein active site. We will begin by covering intramolecular reactions and discussing how the protein activates the substrate for reduction by altering the redox-potential of alkyl halides and templating the charge transfer complex between the substrate and flavin-cofactor. Protein engineering has been used to modify the fundamental photophysics of these reactions, highlighting the opportunity to tune these systems further by using directed evolution. This section highlights the range of coupling partners and radical termination mechanisms available to intramolecular reactions.
The next section will focus on intermolecular reactions and the role of enzyme-templated ternary charge transfer complexes among the cofactor, alkyl halide, and coupling partner in gating electron transfer to ensure that it only occurs when both substrates are bound within the protein active site. We will highlight the synthetic applications available to this activation mode, including olefin hydroalkylation, carbohydroxylation, arene functionalization, and nitronate alkylation. This section also discusses how the protein can favor mechanistic steps that are elusive in solution for the asymmetric reductive coupling of alkyl halides and nitroalkanes. We are aware of several recent EREDs-catalyzed photoenzymatic transformations from other groups. We will discuss results from these papers in the context of understanding the nuances of radical initiation with various substrates.
These biocatalytic asymmetric radical reactions often complement the state-of-the-art small-molecule-catalyzed reactions, making EREDs a valuable addition to a chemist’s synthetic toolbox. Moreover, the underlying principles studied with these systems are potentially operative with other cofactor-dependent proteins, opening the door to different types of enzyme-catalyzed radical reactions. We anticipate that this Account will serve as a guide and inspire broad interest in repurposing existing enzymes to access new transformations.
-
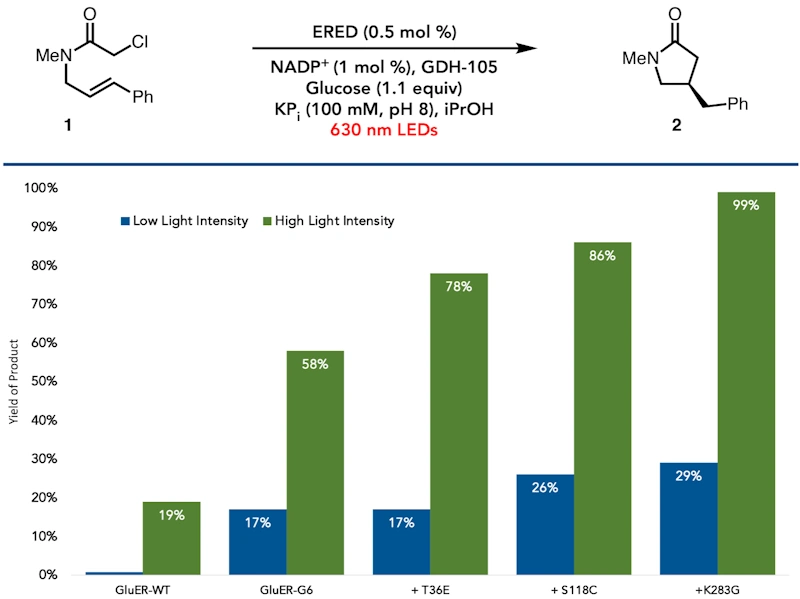
Engineering a Photoenzyme to Use Red Light
ChemRxiv. 2024; doi:10.26434/chemrxiv-2024-cjs5j
Publication Abstract
Photoenzymatic catalysis is an emerging platform for asymmetric synthesis. In most of these reactions, the protein templates a charge transfer complex between the cofactor and substrate, which absorbs in the blue region of the electromagnetic spectrum. Here, we report the engineering of a photoenzymatic ‘ene’-reductase to utilize red light (620 nm) for a radical cyclization reaction. Mechanistic studies indicate that red light ac-tivity is achieved by introducing a broadly absorbing shoulder off the previously identified cyan absorption feature. Molecular dynamics simulations, docking, and excited-state calculations suggest that red light absorption is a 𝜋→ 𝜋* transition from flavin to the substrate, while the cyan feature is the red-shift of the flavin 𝜋→ 𝜋* transition, which occurs upon substrate binding. Differences in the excitation event help to disfavor alkylation of the flavin cofactor, a pathway for catalyst decomposition observed with cyan light but not red.
-
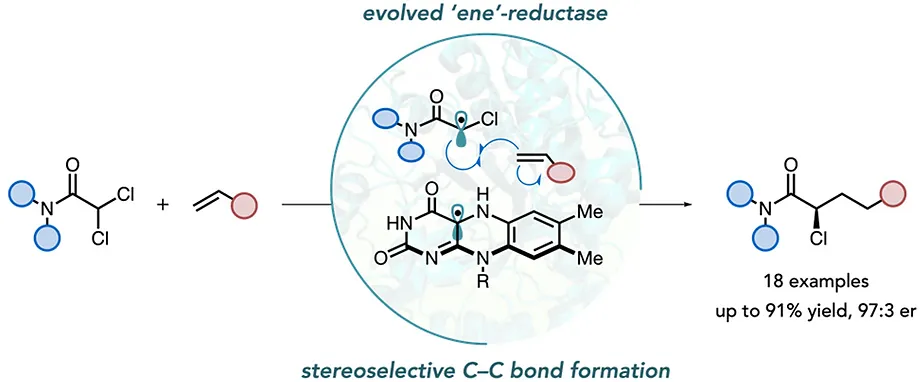
Asymmetric Synthesis of 𝛼-Chloroamides via Photoenzymatic Hydroalkylation of Olefins
J. Am. Chem. Soc. 2024, 146, 11, 7191–7197
Publication Abstract
Photoenzymatic intermolecular hydroalkylations of olefins are highly enantioselective for chiral centers formed during radical termination but poorly selective for centers set in the C–C bond-forming event. Here, we report the evolution of a flavin-dependent “ene”-reductase to catalyze the coupling of α,α-dichloroamides with alkenes to afford α-chloroamides in good yield with excellent chemo- and stereoselectivity. These products can serve as linchpins in the synthesis of pharmaceutically valuable motifs. Mechanistic studies indicate that radical formation occurs by exciting a charge-transfer complex templated by the protein. Precise control over the orientation of molecules within the charge-transfer complex potentially accounts for the observed stereoselectivity. The work expands the types of motifs that can be prepared using photoenzymatic catalysis.

Our team.
The Hyster Lab is led by Principal Investigator, Todd Hyster. Our research team comprises a group of exceptional post-doctoral associates and fellows, graduate students, and undergraduates.

